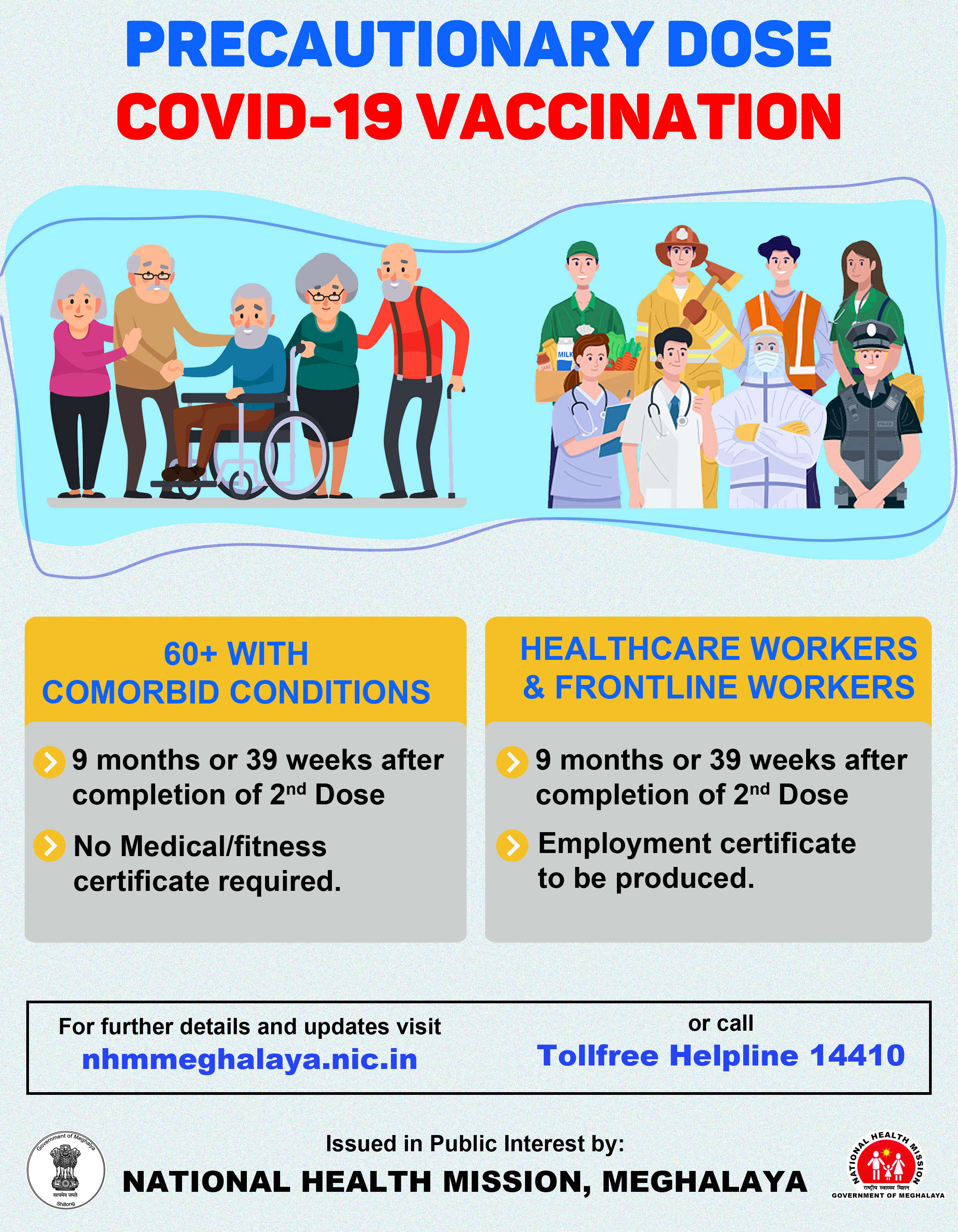
Human trials for COVAXIN gets green light
HEALTH | JULY 20, 2020:
AIIMS panel gives nod for human clinical trials of indigenous vaccine candidate COVAXIN
The AIIMS Ethics Committee has given its nod for a human clinical trial of the indigenously developed COVID-19 vaccine candidate COVAXIN.
AIIMS is likely to begin the exercise by enrolling healthy volunteers from Monday.
ALSO READ:
https://thenortheasttoday.com/india-reports-highest-single-day-spike-of-covid-19-cases/
AIIMS-Delhi is among the 12 sites selected by the Indian Council for Medical Research for conducting phase I and II human trials of COVAXIN. In phase I, the vaccine would be tested on 375 volunteers and a maximum of 100 of them would be from AIIMS.
Healthy volunteers having no comorbid conditions and without a history of COVID-19, aged more than 18 years and less than 55 years, would be eligible to participate in the randomised, double-blind, placebo-controlled clinical trial.